The novel corona passage virus epidemic has resulted in the common people understanding that "nucleic acid detection" technology can quickly and economically detect whether a person has been infected with the novel coronavirus. And this is just a microcosm of the newer iterations of pathogen detection technology.
With the accumulation of data and the development of technology, the application of high-throughput sequencing technology in clinical pathogen detection, epidemiologic investigation and other aspects has become increasingly mature. The mNGS detection method, based on all nucleic acid sequencing, has progressively manifested greater advantages in clinical detection and the discovery of new epidemiological pathogens. mNGS can be used to detect pathogens in the samples unbiasedly, as long as pathogens reaching a certain abundance, while the detection of low-abundant pathogens is not effective (the detection lacks the required sensitivity). In order to achieve sufficient sequencing saturation, the sequencing data volume of 20M reads is commonly used in clinical practice, which makes the detection cost of single samples high. Pathogen detection based on targeted capture can perfectly compensate for these shortcomings.
| |
mNGS |
tNGS(capture probes or ultra-multiplex PCR)) |
| Detection range |
No limit, acceptable beyond detection limit |
Only pathogens designed with probes/primers can be detected, usually tens to hundreds |
| Turn around time |
24h |
24h |
| Sensitivity |
General |
Very high |
| Test fee |
Comparatively high |
Comparatively low |
Table 1 Comparison of mNGS and tNGS .
Targeted capture based pathogen detection (targeted NGS, tNGS, targeted capture and ultra-multiplex PCR) allows for the designing of specific probes and primers according to the sequences of determined pathogenic species, which enriches the pathogenic sequences in the extracted nucleic acids and then sequences them. After the enrichment of nucleic acids, there is a significant reduction in human source background, and the proportion of pathogenic sequences increases from single digits to more than 90%, which can significantly improve detection sensitivity while greatly reducing the amount of sequencing data and analysis workload. Using a rapid library preparation solution, a turn around time similar to that of mNGS can be obtained, which greatly meets the needs of a large number of sample tests such as those in clinical use and epidemiologic investigation.
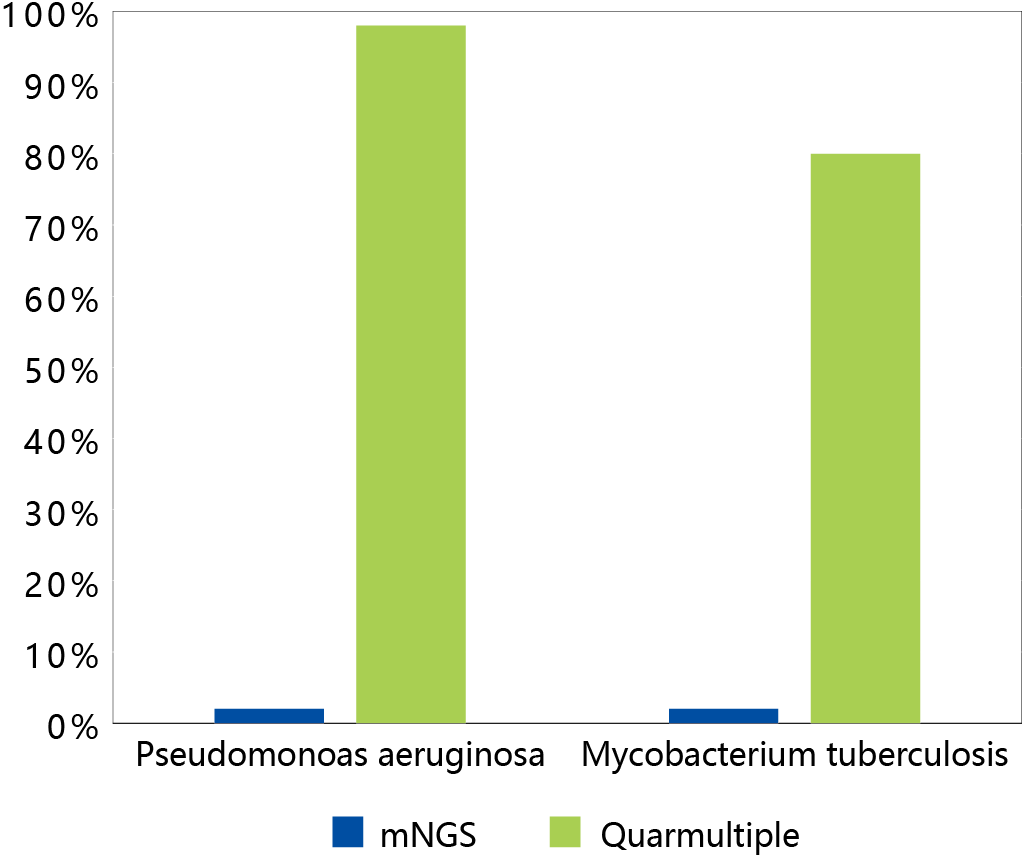
Figure 1 I Comparison the performance of mNGS and ultra-multiplex PCR enrichment (NZ4003A) for two samples. The vertical axis is the proportion of sequences, and the enrichment effect is clearly evident.
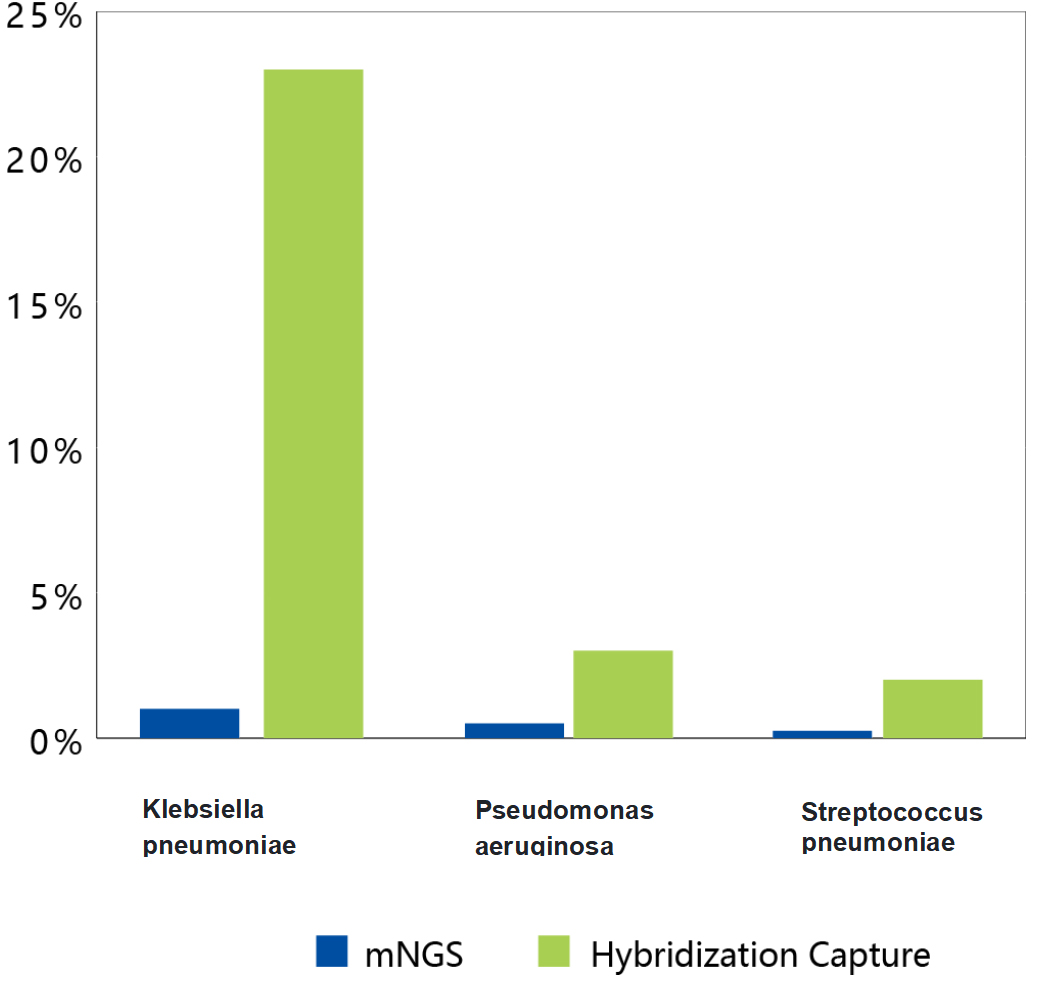
Figure 2 I Comparison of performance using mNGS and liquid phase hybrid capture respectively (NY1023A). Different samples showed enrichment ranging from 10-90X.
Dynegene Technologies is the first company in China which have the ability to independently synthesize oligo pools at high throughput. Based on oligo pool synthesis, liquid probe hybridization capture and multiplex PCR were developed for targeted sequencing. Combined with the company's self-developed algorithm, in consideration of the more genomic variation of pathogenic species, conserved regions within the species are selected and specific primers or probes are designed, which allows for the development of detection products specifically enriched for pathogens. Combined with DNA and RNA co-preparation library products, the DNA and RNA sequences of pathogens can be detected simultaneously. Aimed at different detection ranges and sample conditions, multiplex amplicon and probe hybridization capture have their own focal points and advantages, and Dynegene is in a position to provide suggestions according to specific circumstances to help customers develop the most suitable detection solution.
For the respiratory passage, 76 pathogen detection products and new coronavirus detection products have been available. We warmly welcome you to contact us for consultation and purchase of our existing products, but you also have the option to develop customize your own pathogen detection products according to your individual needs.
| Item No. |
Product Name |
Format |
Description |
| NY1021A |
QuarXeq coVID-19 Virus Probes |
96rxn |
Whole genome sequence capture of the novel coronavirus |
| NY1023A |
QuarXeq Respiratory Pathogens Probes |
96rxn |
Capture probes for 76 respiratory pathogens |
| NZ4003A |
QuarMultiple Respiratory Pathogens Amplicon |
96rxn |
Multiplex primers for 76 respiratory pathogens |
| NK3002A |
QuarPlex 16S rDNA Kit |
96rxn |
116S rDNA assay kit V3-V4 |
Table 2 List of Dynegene products related to pathogen detection
 NGSHybridization Capture DNA Probe QuarStar Human All Exon Probes 4.0 (Tumor) QuarStar Human All Exon Probes 4.0 (Standard) QuarStar Liquid Pan-Cancer Panel 3.0 QuarStar Pan-Cancer Lite Panel 3.0 QuarStar Pan-Cancer Fusion Panel 1.0 QuarStar Pan Cancer Panel 1.0 Hybridization Capture RNA Probe QuarXeq Human All Exon Probes 3.0 HRD panel Library Preparation DNA Library Preparation Kit Fragmentation Reagent mRNA Capture Kit rRNA Depletion Kit QuarPro Superfast T4 DNA Ligase Hybridization Capture QuarHyb Super DNA Reagent Kit QuarHyb DNA Plus 2 Reagent Kit QuarHyb DNA Reagent Kit Plus QuarHyb One Reagent Kit QuarHyb Super Reagent Kit Pro Dynegene Adapter Family Dynegene Blocker Family Multiplex PCR QuarMultiple BRCA Amplicon QuarMultiple PCR Capture Kit 2.0 PathoSeq 450 Pathogen Library Corollary Reagent Streptavidin magnetic beads Equipment and Software The iQuars50 NGS Prep System
NGSHybridization Capture DNA Probe QuarStar Human All Exon Probes 4.0 (Tumor) QuarStar Human All Exon Probes 4.0 (Standard) QuarStar Liquid Pan-Cancer Panel 3.0 QuarStar Pan-Cancer Lite Panel 3.0 QuarStar Pan-Cancer Fusion Panel 1.0 QuarStar Pan Cancer Panel 1.0 Hybridization Capture RNA Probe QuarXeq Human All Exon Probes 3.0 HRD panel Library Preparation DNA Library Preparation Kit Fragmentation Reagent mRNA Capture Kit rRNA Depletion Kit QuarPro Superfast T4 DNA Ligase Hybridization Capture QuarHyb Super DNA Reagent Kit QuarHyb DNA Plus 2 Reagent Kit QuarHyb DNA Reagent Kit Plus QuarHyb One Reagent Kit QuarHyb Super Reagent Kit Pro Dynegene Adapter Family Dynegene Blocker Family Multiplex PCR QuarMultiple BRCA Amplicon QuarMultiple PCR Capture Kit 2.0 PathoSeq 450 Pathogen Library Corollary Reagent Streptavidin magnetic beads Equipment and Software The iQuars50 NGS Prep System Primers and Probes
Primers and Probes RNA SynthesissgRNA miRNA siRNA
RNA SynthesissgRNA miRNA siRNA



 Gene Synthesis
Gene Synthesis Oligo Pools
Oligo Pools CRISPR sgRNA Library
CRISPR sgRNA Library Antibody Library
Antibody Library Variant Library
Variant Library





 Tel: 400-017-9077
Tel: 400-017-9077 Address: Floor 2, Building 5, No. 248 Guanghua Road, Minhang District, Shanghai
Address: Floor 2, Building 5, No. 248 Guanghua Road, Minhang District, Shanghai Email:
Email: